- A study led by the National Oceanography Centre (NOC) has evaluated a potential way to remove CO2 from the atmosphere using the ocean.
- Scientists used a state-of-the-art climate model to simulate the benefits of depositing olivine (a bright green mineral) sands on the seafloor.
- This process is known as ocean alkalinity enhancement (OAE) and could be a future approach for increasing CO2 uptake by the ocean and reducing the impact of ocean acidification.
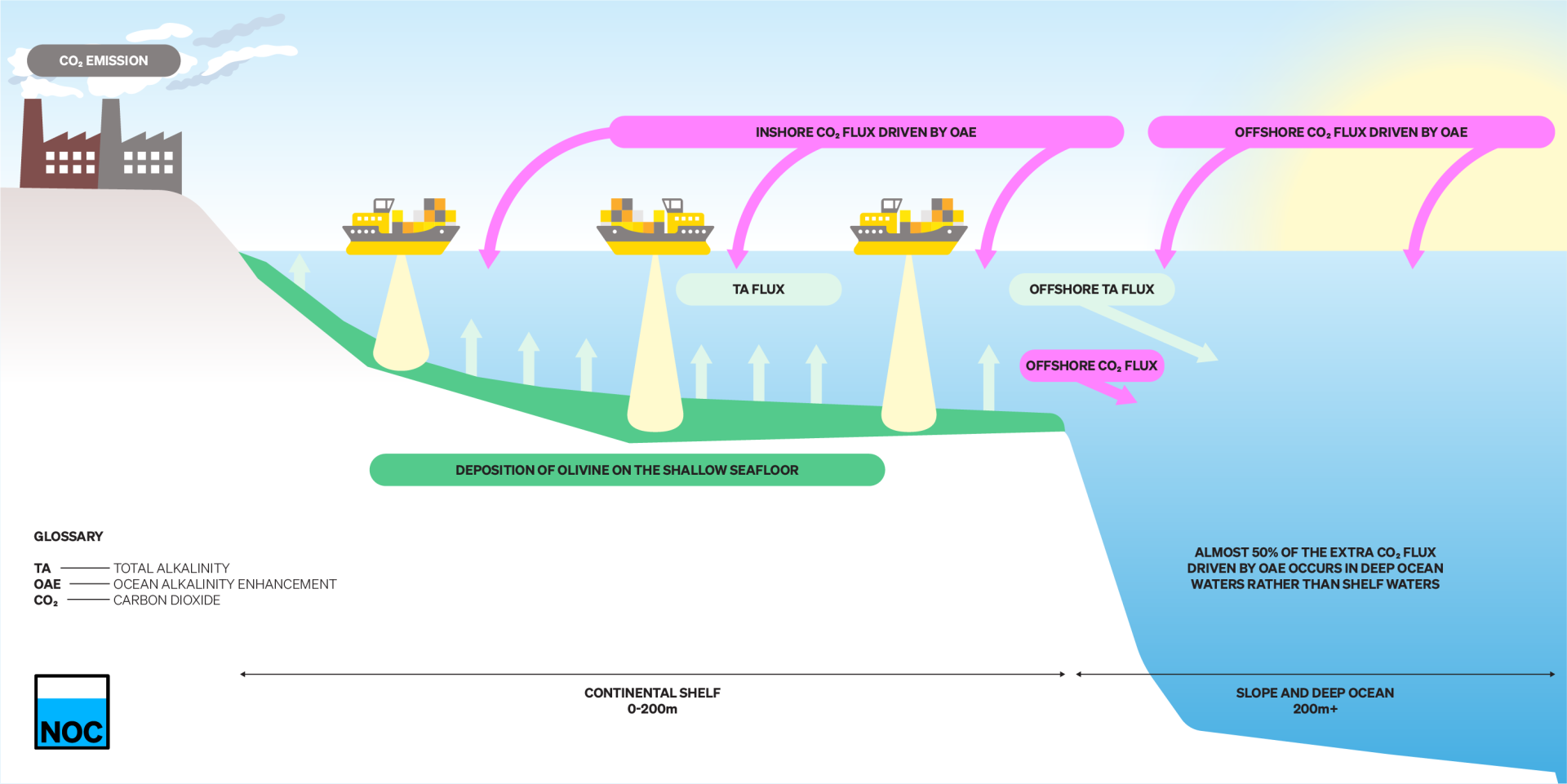
- Results included the finding that almost half of the CO2 taken up happened away from OAE operations, and that land reservoirs of carbon were also affected.
- Though the study found OAE was effective at drawing more CO2 into the ocean, scientists say more research will be needed to understand the wider impacts of the method.
- You can read the open-access paper in Earth's Future Journal.
Scientists from the National Oceanography Centre (NOC) have evaluated a potential new way to remove CO2 from the atmosphere, by adding a common mineral called olivine to the ocean.
In a paper published in Earth’s Future journal, scientists found that adding olivine helped to absorb additional CO2 as it dissolved on the bottom of the ocean, ultimately increasing the uptake of CO2 by 8% over the 21st century. This increase equates to approximately two years’ worth of present-day, global CO2 emissions.
NOC scientists, Dr. Julien Palmiéri and Dr. Andrew Yool, employed a state-of-the-art climate model to simulate the benefits of depositing olivine, a bright green mineral, on the seafloor to change the chemical make-up of the ocean – increasing its capacity to act as a carbon sink. This process is known as ocean alkalinity enhancement (OAE) and could be a future approach for increasing CO2 uptake by the ocean and reducing the impact of ocean acidification.
The team ran a future projection simulation which spanned 2020 to 2100 and involved depositing 81 billion metric tonnes of olivine into shallow areas of the ocean. Shallow waters are important since the chemical products of the dissolving olivine need to be near the ocean’s surface so that the extra CO2 is successfully absorbed from the atmosphere.
Dr Andrew Yool, an ocean biogeochemistry modeller and co-lead of the study, said: “What this research shows is that there is potential in utilising the ocean to absorb CO2 and help support the drive to reach net zero. However, the research also makes clear that this is not a silver bullet and there are limitations on the scale it can be deployed at. Keeping global temperature rise below 2°C as specified by the Paris Agreement will still require a significant reduction in emissions”.
Significantly, while the olivine was added only to coastal and shallow ocean areas during the simulation, the paper found that around half of all the extra CO2 absorption occurred away from the areas where the olivine was being added. This finding means that significant fieldwork would likely be needed in order to effectively monitor and verify its efficiency.
Speaking further on the study, Dr Yool, added: “More research will need to be commissioned to understand some of the wider impacts of OAE, such as the carbon impact of mining the additional olivine that would be needed. Additionally, any practical deployment of olivine in the ocean will need to carefully monitor the impact on sea life.”
The paper, titled 'Global-scale evaluation of coastal ocean alkalinity enhancement in a fully-coupled Earth system model', used UKESM1, the UK’s Earth system model, for its future simulations. The ocean carbon cycle of UKESM1 makes use of MEDUSA, the marine biogeochemistry submodel developed and operated by the National Oceanography Centre. MEDUSA simulates the ocean side of the wider carbon cycle, including the atmosphere and land, and permits the full climate effects of the olivine scheme to be examined.
How does OAE work?
- Olivine sands are deposited to the shallow seafloor of the continental shelves.
- These dissolve and add alkalinity (TA) to the overlying shelf waters that are well-mixed and in contact with the surface.
- This added TA raises the buffering capacity of seawater and leads to additional CO2 absorption from the atmosphere.
- OAE acts this way to raise the ocean’s concentrations of both TA and dissolved CO2 (DIC).
- Before some of the TA has absorbed CO2 in the shelves, it is transported by ocean currents into the deep ocean.
- In this study, almost 50% of the additional CO2 absorption from the atmosphere driven by OAE occurs in the surface waters of the deep ocean.
- This makes monitoring and verifying the success of OAE challenging since much of the extra CO2 absorption happens away from where OAE is being applied.